In trying to explain why atoms form bonds, G. N. Lewis proposed that an atom is most stable if its outer shell is either filled or contains eight electrons and it has no electrons of higher energy. According to Lewis’s theory, an atom will give up, accept, or share electrons in order to achieve a filled outer shell or an outer shell that contains eight electrons. This theory has come to be called the octet rule.
Lithium (Li) has a single electron in its 2s atomic orbital. If it loses this electron, the lithium atom ends up with a filled outer shell—a stable configuration. Removing an electron from an atom takes energy—called the ionization energy. Lithium has a relatively low ionization energy—the drive to achieve a filled outer shell with no electrons of higher energy causes it to lose an electron relatively easily. Sodium (Na) has a single electron in its 3s atomic orbital. Consequently, sodium also has a relatively low ionization energy because, when it loses an electron, it is left with an outer shell of eight electrons. Elements (such as lithium and sodium) that have low ionization energies are said to be electropositive—they readily lose an electron and thereby become
positively charged. The elements in the first column of the periodic table are all electropositive—each readily loses an electron because each has a single electron in its outermost shell.
Electrons in inner shells (those below the outermost shell) are called core electrons. Core electrons do not participate in chemical bonding. Electrons in the outermost shell are called valence electrons, and the outermost shell is called the valence shell. Carbon, for example, has two core electrons and four valence electrons.
Lithium and sodium each have one valence electron. Elements in the same column of the periodic table have the same number of valence electrons, and because the number of valence electrons is the major factor determining an element’s chemical properties, elements in the same column of the periodic table have similar chemical properties. Thus, the chemical behavior of an element depends on its electronic configuration.
When we draw the electrons around an atom, as in the following equations, core electrons are not shown; only valence electrons are shown. Each valence electron is shown as a dot. Notice that when the single valence electron of lithium or sodium is removed, the resulting atom—now called an ion—carries a positive charge.
Fluorine has seven valence electrons (Table 1.2). Consequently, it readily acquires an electron in order to have an outer shell of eight electrons. When an atom acquires an electron, energy is released. Elements in the same column as fluorine (e.g., chlorine, bromine, and iodine) also need only one electron to have an outer shell of eight, so they, too, readily acquire an electron. Elements that readily acquire an electron are said
to be electronegative—they acquire an electron easily and thereby become negatively charged.
Ionic Bonds
Because sodium gives up an electron easily and chlorine acquires an electron readily, when sodium metal and chlorine gas are mixed, each sodium atom transfers an electron to a chlorine atom, and crystalline sodium chloride (table salt) is formed as a result. The positively charged sodium ions and negatively charged chloride ions are independent species held together by the attraction of opposite charges. A bond is an attractive force between two atoms. Attractive forces between opposite charges are called electrostatic attractions. A bond that is the result of only electrostatic attractions is called an ionic bond. Thus, an ionic bond is formed when there is a transfer of electrons, causing one atom to become a positively charged ion and the other to become a negatively charged ion.
Sodium chloride is an example of an ionic compound. Ionic compounds are formed when an element on the left side of the periodic table (an electropositive element) transfers one or more electrons to an element on the right side of the periodic table (an electronegative element).
Covalent Bonds
Instead of giving up or acquiring electrons, an atom can achieve a filled outer shell by sharing electrons. For example, two fluorine atoms can each attain a filled shell of eight electrons by sharing their unpaired valence electrons. A bond formed as a result of sharing electrons is called a covalent bond.
Two hydrogen atoms can form a covalent bond by sharing electrons. As a result of covalent bonding, each hydrogen acquires a stable, filled outer shell (with two electrons).
Similarly, hydrogen and chlorine can form a covalent bond by sharing electrons. In doing so, hydrogen fills its only shell and chlorine achieves an outer shell of eight electrons.
A hydrogen atom can achieve a completely empty shell by losing an electron. Loss of its sole electron results in a positively charged hydrogen ion. A positively charged hydrogen ion is called a proton because when a hydrogen atom loses its valence electron, only the hydrogen nucleus—which consists of a single proton—remains. A hydrogen atom can achieve a filled outer shell by gaining an electron, thereby forming a negatively charged hydrogen ion, called a hydride ion.
Because oxygen has six valence electrons, it needs to form two covalent bonds to achieve an outer shell of eight electrons. Nitrogen, with five valence electrons, must form three covalent bonds, and carbon, with four valence electrons, must form four covalent bonds to achieve a filled outer shell.
Polar Covalent Bonds
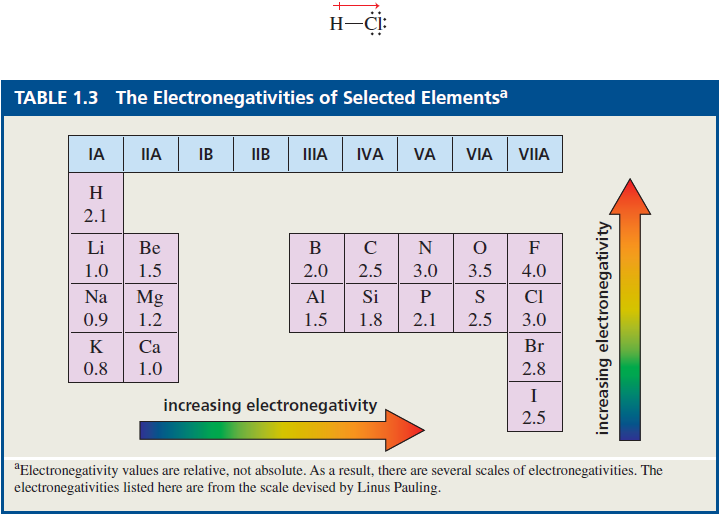
In the F - F and H -H covalent bonds shown previously, the atoms that share the bonding electrons are identical. Therefore, they share the electrons equally; that is, each electron spends as much time in the vicinity of one atom as in the other. An even (nonpolar) distribution of charge results. Such a bond is called a nonpolar covalent bond. In contrast, the bonding electrons in hydrogen chloride, water, and ammonia are more attracted to one atom than another because the atoms that share the electrons in these molecules are different and have different electronegativities. Electronegativity is the tendency of an atom to pull bonding electrons oward itself. The bonding electrons in hydrogen chloride, water, and ammonia molecules are more attracted on the atom with the greater electronegativity. This results in a polar distribution of charge. A polar covalent bond is a covalent bond between atoms of different electronegativities. The electronegativities of some of the elements are shown in Table 1.3. Notice that electronegativity increases as you go from left to right across a row of the periodic table or up any of the columns. A polar covalent bond has a slight positive charge on one end and a slight negative charge on the other. Polarity in a covalent bond is indicated by the symbols (S+) and which denote partial positive and partial negative charges, respectively. The negative end of the bond is the end that has the more electronegative atom. The greater the difference in electronegativity between the bonded atoms, the more polar the bond will be.
The direction of bond polarity can be indicated with an arrow. By convention, the
arrow points in the direction in which the electrons are pulled, so the head of the arrow
is at the negative end of the bond; a short perpendicular line near the tail of the arrow
marks the positive end of the bond.
You can think of ionic bonds and nonpolar covalent bonds as being at the opposite ends of a continuum of bond types. An ionic bond involves no sharing of electrons. A nonpolar covalent bond involves equal sharing. Polar covalent bonds fall somewhere in between, and the greater the difference in electronegativity between the atoms forming the bond, the closer the bond is to the ionic end of the continuum. C - H bonds are
relatively nonpolar, because carbon and hydrogen have similar electronegativities (electronegativity difference = 0.4 see Table 1.3). N - H bonds are relatively polar (electronegativity differnece = 0.9 ), but not as polar as O - H bonds (electronegativity differnece = 1.4). The bond between sodium and chloride ions is closer to the
ionic end of the continuum (electronegativity difference = 2.1 ), but sodium chloride is not as ionic as potassium fluoride (electronegativity difference = 3.2).










No comments:
Post a Comment